how are atoms similar to elements ,relationship between atom and element,how are atoms similar to elements, Both atoms and elements are closely related. An atom is a part of the element and is sometimes available in free form. While the element is . Discover new nail colors, perfumes, moisturizers, and more at chanel.com. View the latest fragrance, makeup and skincare products. Discover new nail colors, perfumes, moisturizers, and more at chanel.com. Main content; Main navigation; Enable high contrast; Menu - main navigation; . Shop online. Eyewear. Go back to main navigation .
The relationship between atoms and elements is foundational to understanding chemistry and the structure of matter. These two terms are often used interchangeably, but they represent distinct concepts in the scientific world. To understand their differences, it's essential to delve into what makes up an atom, what defines an element, and how these concepts interact with each other.
Defining Atoms and Elements
Before diving into how atoms and elements are similar, let’s start with the basic definitions of both terms:
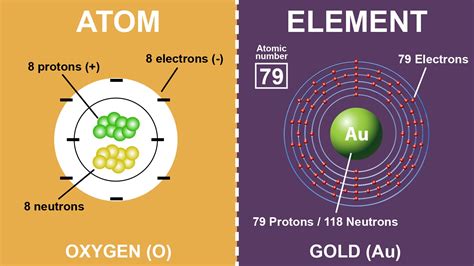
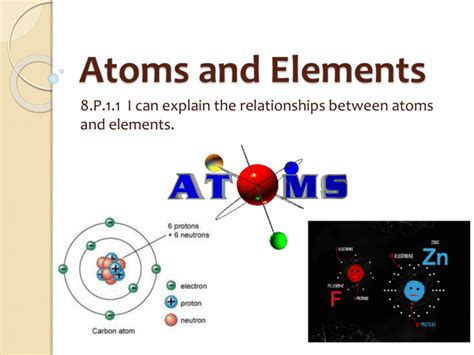
- Atom: An atom is the smallest unit of matter that retains the properties of an element. It consists of a nucleus (composed of protons and neutrons) surrounded by a cloud of electrons. The number of protons in the nucleus of an atom defines which element it is and determines its chemical properties.
- Element: An element is a pure substance consisting of only one type of atom. The elements are organized on the Periodic Table according to their atomic number, which corresponds to the number of protons in the nucleus of their atoms. Each element has distinct chemical properties, which arise from the behavior of its atoms.
At first glance, atoms and elements seem quite similar, especially since atoms are the building blocks of elements. However, they are not the same thing. To understand the nuances of their relationship, let's break down the differences and explore their similarities in detail.
Distinguishing Between Atoms and Elements
Atomic Structure vs. Chemical Identity
The key difference between atoms and elements lies in their definition and scope. Atoms are individual particles made up of protons, neutrons, and electrons. They are the fundamental units of all matter. On the other hand, an element refers to a collection of atoms with the same number of protons in their nuclei.
Each atom, no matter how small, can exist independently or combine with other atoms to form molecules. An element, however, is a pure substance that consists entirely of atoms with the same atomic number. For instance, a single hydrogen atom (H) is an atom, but when you collect many hydrogen atoms, you get the element hydrogen.
Atomic Number and Chemical Properties
The number of protons in the nucleus of an atom is called its atomic number. This number defines what element an atom belongs to. For example, an atom with one proton is hydrogen (atomic number 1), while an atom with six protons is carbon (atomic number 6). Thus, an element is essentially a classification of atoms that share the same atomic number.
An element’s properties are directly linked to the arrangement and behavior of its atoms. For instance, the element oxygen (O) consists of atoms with eight protons, and this atomic structure gives oxygen its distinctive chemical properties, such as its ability to form bonds with other elements and its role in respiration.
Elemental Forms vs. Atomic Forms
Atoms can exist in isolation, but elements are usually encountered in the form of molecules, which are composed of two or more atoms bonded together. For example, the element oxygen exists as O₂, a molecule made up of two oxygen atoms. Similarly, nitrogen (N₂) is a diatomic molecule, which means that its elemental form consists of two nitrogen atoms.
This distinction is important because while an atom is the basic unit of matter, an element can be seen as a collection of atoms that share the same characteristics.
Comparing and Contrasting Atoms and Elements
Atoms as Building Blocks of Elements
Atoms are the smallest possible units of matter that maintain the properties of an element. In fact, elements themselves are collections of atoms with identical atomic numbers. Therefore, atoms are the fundamental building blocks of elements.
However, atoms can combine in different ways to form compounds, and this process often results in entirely new properties. For instance, the element oxygen is made up of oxygen atoms, but when two oxygen atoms combine to form O₂, it becomes a colorless, odorless gas that plays a crucial role in respiration and combustion.
Elements as Collections of Identical Atoms
An element is, at its core, a collection of atoms that are all the same type, meaning they have the same number of protons in their nuclei. This uniformity gives an element its distinctive properties. For example, all iron (Fe) atoms have 26 protons, and this consistent atomic structure imparts iron with its well-known characteristics, such as its magnetic properties and its ability to form alloys with other metals.
Thus, while atoms are the fundamental unit, elements are composed of atoms that are indistinguishable from each other. The relationship between atoms and elements can be visualized as a building block analogy: atoms are the bricks, and elements are the structures made up of those bricks.
Atom vs. Element Diagram
how are atoms similar to elements $11K+
how are atoms similar to elements - relationship between atom and element